Global alarm as new monkeypox (mpox) strain fuels surge across Africa and beyond
Few days ago, the WHO declared international emergency amid rapid spread of the new mpox virus strain.
On August 14, the WHO director general, Dr. Tedros Adhanom Ghebreyesus, declared the recent surge of monkeypox (mpox) cases in the Democratic Republic of the Congo (DRC) and other African countries a public health emergency of international concern. This decision follows recommendations from an independent expert committee, highlighting the rapid spread of a newly mutated lineage of mpox, and its detection in neighboring countries like Burundi, Kenya, Rwanda, and Uganda.
So called clades can be used to classify different viral lineages or strains based on genetic sequences. There are two main clades of the mpox virus. Clade 1, which presumably causes more severe disease, and clade 2, which was responsible for the 2022 global outbreak. A new offshoot, clade 1b, emerged in the DRC in 2023 and is spreading among people through close contact rather than the typical zoonotic spread (from animals to humans). Studies are ongoing to better understand this new clade.

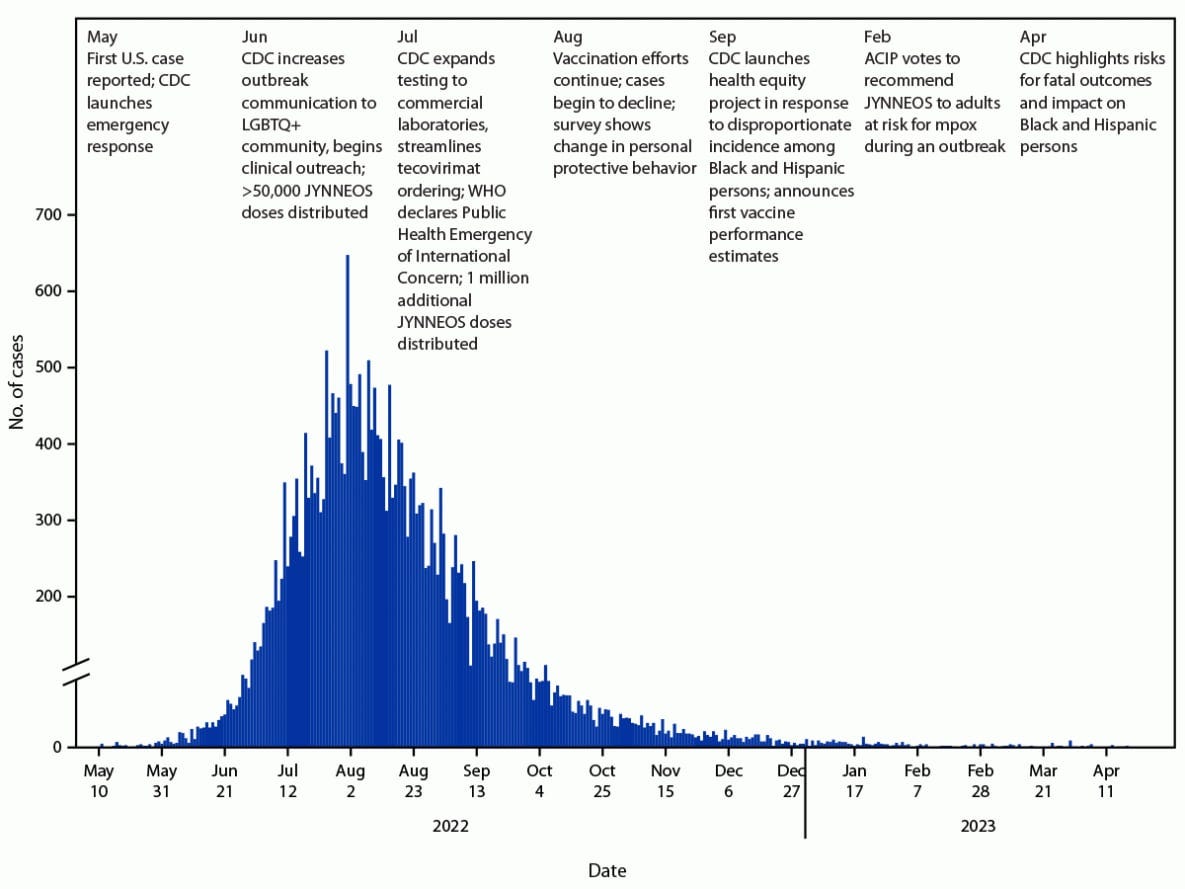
The mpox outbreak in 2022 was a significant global health event, with cases reported in over 100 countries worldwide. It primarily affected West and Central African countries where mpox is endemic, such as Nigeria, DRC, and Cameroon. But as the disease spread, nearly 100,000 cases and 200 deaths were reported in more than 100 non-endemic countries, including the U.S., Canada, Mexico, and countries across Europe, South America, and Asia.
The 2022 outbreak resulted in about 32,000 confirmed cases and 58 deaths in the U.S. and it highlighted the potential for mpox to spread globally and the need for improved surveillance, prevention, and treatment strategies.
The new clade 1b appears to transmit more easily between people compared to other clades (1a and 2). This heightened transmissibility could lead to faster spread within communities and across borders. It is causing more severe disease, with higher rates of complications and death. It also exhibits multiple mutations in genes associated with virulence, host recognition, and immune evasion compared to other clades.
As of August 2024, the WHO reported over 15,600 mpox cases and 537 deaths in the DRC alone, surpassing last year’s total. The current mortality rate remains under 4%. For comparison, the mortality rate of clade 2 that caused the 2022 outbreak was less than 0.2%.
The current situation is particularly concerning in densely populated camps for displaced people around DRC’s capital, Goma, where sexual violence is rampant. Limited resources for identifying, monitoring, and treating cases, along with a lack of vaccines, exacerbate the crisis.
The spread of the new mpox strain to Sweden reported on August 15, 2024, has heightened concerns about its potential spread across Europe due to frequent travel between the continent and Africa. This incidence marked the first instance of this mpox clade 1b being found outside Africa.
The European Center for Disease Prevention and Control has raised the risk level for Europe from “very low” to “low” and advised increased vigilance and preparedness. European countries are enhancing public health measures and preparing for potential cases while also supporting Africa with vaccine donations to curb the spread.
What is mpox?
Mpox is a viral zoonotic (transmitted from animals to humans) disease caused by the monkeypox virus, which belongs to the Poxviridae family of viruses. The virus was originally called “monkeypox” because it was first discovered in laboratory monkeys in 1958 but monkeys are not the primary hosts of the virus. Unlike smallpox, which only infects humans, mpox can infect a variety of animals including small mammals, primates, and rodents.
Mpox exhibits symptoms that bear similarities to those of smallpox. Fever, headache, muscle aches, backache, swollen lymph nodes, chills, and a characteristic rash are common to both diseases. But smallpox is generally more severe than monkeypox, with a higher mortality rate. The smallpox vaccine provides protection against monkeypox, but its effectiveness has decreased over time as vaccination programs were discontinued after smallpox eradication.
While the lesions may look somewhat similar to chickenpox and the two have some overlapping symptoms, they are caused by different viruses and are not closely related. For example, mpox lesions are larger and generally deeper and more painful.
Mpox rash starts with flat red spots that develop into raised bumps, fluid-filled blisters, and eventually scabs. Lesions usually affect the face, arms, legs, and trunk. Complications can include pneumonia, encephalitis, and sepsis which can be fatal if not properly managed.
Monkeypox is transmitted to humans through close contact with infected animals, their excretions or secretions. Human-to-human transmission occurs through prolonged direct or close contact, or through blood, body fluids, or mucosal lesions. The virus can also spread through contaminated materials such as bedding.
Epidemiology and spread
Mpox was historically confined to Central and West Africa. However, the major outbreak in 2022 mainly occurred for two reasons:
Waning immunity from smallpox vaccination since smallpox was eradicated in 1980.
Increased human-to-human transmission, especially among men who have sex with men, sex workers and close household contacts.
The basic reproduction number (R0) for monkeypox ranges from 0.6 to 1.1, indicating moderate transmissibility. The case fatality rate is estimated to be around 3-6%.
The groups most at risk are newborn babies, children, people who are pregnant and people with underlying immune deficiencies such as from advanced HIV disease may be at higher risk of more serious mpox disease and death.
Vaccines and treatment
Two vaccines are approved for prevention of monkeypox. JYNNEOS (also known as Imvamune or Imvanex) and ACAM2000.
In April 2024, Bavarian Nordic announced the commercial launch of its FDA-approved mpox vaccine, JYNNEOS, in the U.S., marking a significant expansion in access, allowing healthcare providers to order the vaccine through standard distribution channels for at-risk individuals at pharmacies and clinics.
JYNNEOS is a live, non-replicating vaccine developed for the prevention of both smallpox and monkeypox. Unlike traditional smallpox vaccines, JYNNEOS does not cause a significant skin lesion at the injection site, which reduces the risk of inadvertent inoculation.
It has demonstrated good immunogenicity, with a high rate of seroconversion observed in clinical trials. Serious adverse events are rare, making it a safer option compared to older vaccines (like ACAM2000), particularly for immunocompromised individuals and pregnant women.
The CDC now recommends routine use of JYNNEOS for those 18 and older with certain risk factors, following updated guidance in October 2023. Bavarian Nordic, leveraging its long-standing partnership with the U.S. government, aims to increase vaccine accessibility and awareness, particularly within high-risk communities.
Emergent BioSolutions’ ACAM2000 is a live, replicating vaccine that was approved by the FDA in 2007 for the prevention of smallpox. It is derived from the vaccinia virus and has been used in the U.S. Strategic National Stockpile.
This vaccine is administered via scarification, leading to a localized skin reaction that indicates a successful vaccination. However, this method poses a risk of transmission through contact with the vaccination site.
Opportunities in monkeypox response
As the monkeypox outbreak continues to pose public health challenges, the biotech sphere has several avenues to explore in order to contribute to the global response.
1. Vaccine development:
Enhancing existing vaccines: improving the current vaccines like JYNNEOS and ACAM2000 by developing formulations that are easier to store and distribute, or that require fewer doses.
mRNA vaccine platforms: following the success of mRNA vaccines for COVID-19, companies like BioNTech are exploring mRNA technology for monkeypox vaccines.
2. Antiviral drug development:
Repurposing existing antivirals: investigate the efficacy of existing antiviral drugs, such as tecovirimat, brincidofovir, and cidofovir, for treating monkeypox. This approach can expedite the availability of therapies since these drugs have already undergone some level of safety testing.
Novel antiviral research: there is a need for new antiviral agents specifically targeting the monkeypox virus.
3. Diagnostics and testing:
Rapid diagnostic tests: Developing rapid and accurate diagnostic tests for monkeypox can help in early detection and containment of outbreaks. Companies can innovate point-of-care testing solutions that provide quick results, facilitating timely public health interventions.
Surveillance tools: Biotech firms can create tools that enhance surveillance of monkeypox outbreaks, utilizing genomic sequencing and data analytics to track the spread and evolution of the virus.
Future outlook
While monkeypox is concerning, experts believe it is unlikely to cause a pandemic like COVID-19. However, continued global surveillance and public health measures are important to control the current outbreak.
Treatment for monkeypox is mainly supportive, with antivirals like tecovirimat (SIGA Technologies) used in severe cases. As mentioned in the previous section, more research is needed to develop better preventive and treatment methods.
To tackle the escalating mpox epidemic in DRC, a dynamic and context-specific response is essential. Immediate vaccine deployment is important to shield high-risk populations, such as healthcare workers and displaced individuals, although current supplies are lacking.
The availability of mpox vaccines, particularly the JYNNEOS vaccine, is extremely limited. The Africa Centers for Disease Control and Prevention estimates that at least 10 million doses are needed to respond effectively to the ongoing epidemic, but supply remains critically low in the affected regions.
Countries that possess stockpiles of vaccines are being urged to donate doses, but negotiations and logistics for these donations can be complex and slow. Additionally, the cost of the JYNNEOS vaccine is too high for many low- and middle-income countries, including DRC. This financial barrier limits the ability of the DRC government and health organizations to procure vaccines independently.
To halt the outbreak, a unified effort is required, focusing on health promotion, community engagement, surveillance, and equitable resource distribution to the most affected areas.
DISCLAIMER: This content is for informational purposes only. It should not be taken as legal, tax, investment, financial, or other advice. The views expressed here are my own and do not reflect the opinions of any company or institution.
DISCLOSURE: I have no business relationships with any company mentioned in this article.