Welcome to This Week in Biotech by Biotech Blueprint, your weekly dose of the latest biotech breakthroughs, clinical trial updates, pharma company news, and forecasts of what’s on the horizon.
MARKET UPDATES
🔹 Biotech ETFs have largely rebounded to their levels before the significant drop earlier this month, reflecting broader market stabilization. This recovery suggests that the biotech sector, often sensitive to economic changes, is stabilizing along with the market. For instance, the iShares Biotechnology ETF (IBB) is now trading around $148 per share, matching its July 2024 levels, indicating a return to more stable conditions for biotech investments.
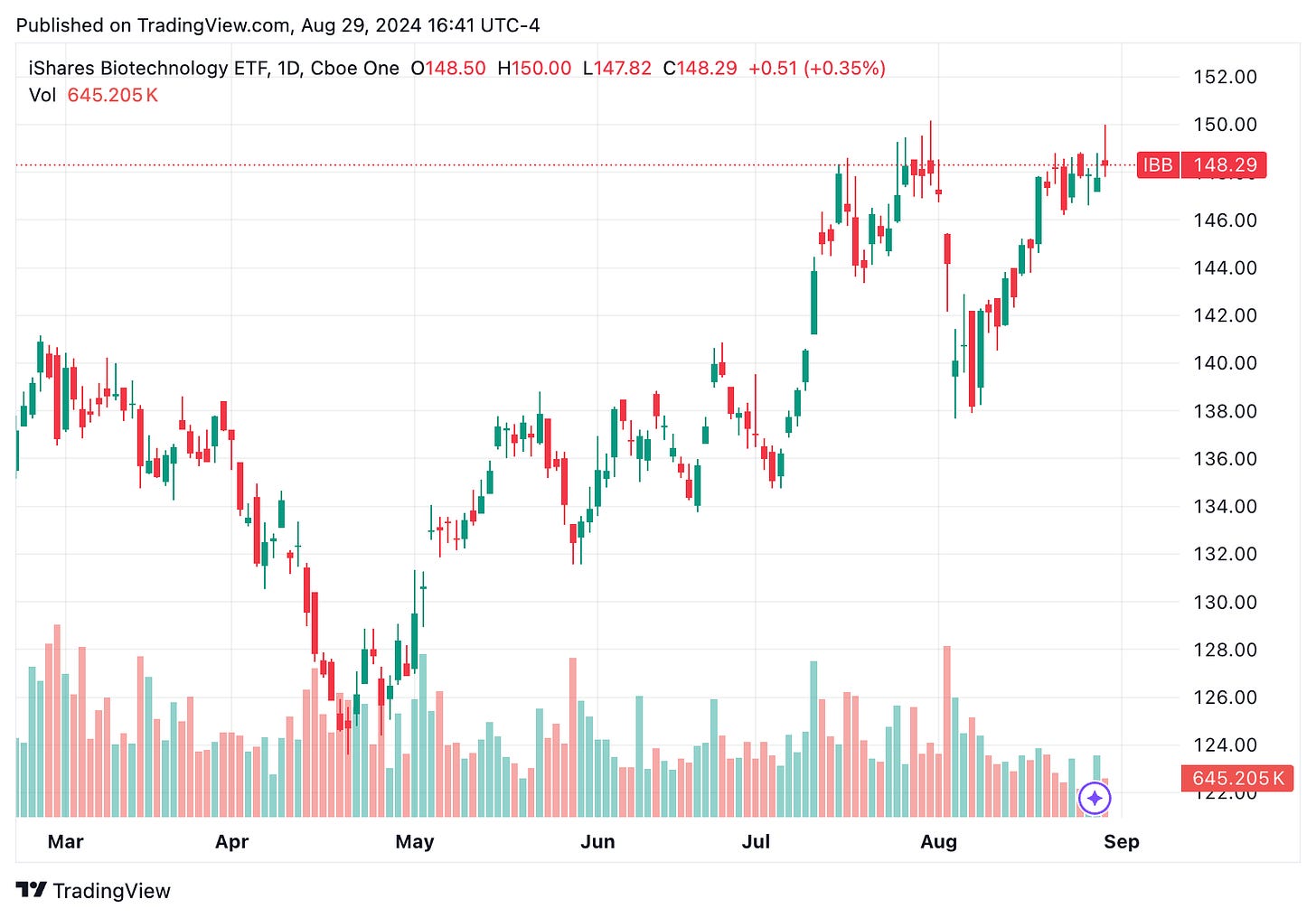
📈Key stock market movements and highlights of the week:📉
🔹 On August 29, BioCardia’s (BCDA) stock surged nearly 83% in pre-market trading after the FDA granted clearance for its Morph DNA Steerable Introducer product family designed for various cardiovascular procedures.
🔹 On August 28, Palatin Technologies’ stock rose approximately 10% following an update on its phase 3 clinical trial for PL9643, a drug candidate aimed at treating dry eye disease. The company reported that the trial met its primary endpoint, demonstrating a statistically significant improvement in symptoms compared to placebo.
🔹 On August 28, Neurocrine Biosciences (NBIX) announced mixed results on its experimental drug NBI-1117568 for treating adults with schizophrenia. Neurocrine’s stock fell 18% premarket on Wednesday, suggesting that investors may have expected stronger results.
🔹 Ensysce Biosciences’ (ENSC) stock surged almost 50% following the announcement that the company received a $14M federal grant from the NIH. This grant is designated for the clinical development of Ensysce’s novel opioid formulation, which is designed to provide pain relief while incorporating overdose protection features.
🔹 Cosciens Biopharma (CSCI) stock plummeted approximately 31% after the company announced on August 27 that its lead drug candidate macimorelin failed to meet the primary endpoint in a phase 3 trial for diagnosing childhood-onset growth hormone deficiency (CGHD). Based on topline results from the DETECT trial, macimorelin did not achieve the study’s main efficacy goal compared to standard growth hormone stimulation tests.
🔹 Despite Invivyd’s (IVVD) announcement on August 27 that its COVID-19 monoclonal antibody treatment pemivibart (PEMGARDA) demonstrated an 84% relative risk reduction in symptomatic COVID-19 compared to placebo in an exploratory analysis, the company’s stock price dropped 29% following the announcement. While the 84% risk reduction in symptomatic COVID-19 is a positive result for Invivyd’s treatment, the stock decline suggests investors may have expected stronger data or are concerned about other factors impacting the company’s outlook.
🔹 Eli Lilly’s (LLY) decision to reduce the cost of certain dosage strengths of its obesity medication Zepbound (tirzepatide) on August 27 has led to a decline in the stock prices of other companies developing weight loss drugs. Viking Therapeutics (VKTX) saw the largest drop, approximately 7%, as it develops a similar GLP-1/GIP dual agonist, VK2735. This could represent a VKTX stock buying opportunity.
🔹 On August 27, Lilly also launched vials for its obesity drug Zepbound. Previously available only as injectable pens, the new vials are more affordable and quicker to produce. As a result, shares of Hims & Hers (HIMS), a leading telehealth company offering compounded GLP-1 drugs, dropped over 7%.
🔹 Vericel Corporation (VCEL) announced on Monday, August 26, that the FDA approved its MACI Arthro treatment for the repair of cartilage defects in the knee. The approval of MACI Arthro represents a significant milestone for Vericel that led to 13% increase in stock price on 5-day basis.
BIOTECH NEWS
🔹 On August 29, BioCardia has received FDA clearance to market its Morph DNA Steerable Introducer product family. These devices, designed for various cardiovascular procedures, allow for the introduction of medical instruments into the vasculature.
🔹 Pfizer has launched PfizerForAll, a digital platform aimed at simplifying healthcare management for Americans. The platform provides same-day access to healthcare professionals, home delivery of medications and diagnostic tests, vaccine appointment scheduling, and support for Pfizer medication costs. It integrates with existing healthcare systems and partners to make health services more accessible, reducing complexity and time for patients.
🔹 It was announced on August 28 that Flagship Pioneering and Quotient Therapeutics have entered a collaboration under a strategic partnership with Pfizer to identify new targets for cardiovascular and renal diseases. This partnership will use Quotient’s somatic genomics platform to analyze genetic mutations in patient tissues to develop potential therapies. Pioneering Medicines, Flagship’s drug development unit, will lead the research efforts.
🔹 As of August 27, Eli Lilly is reducing the prices of its weight loss drug Zepbound for its two lowest doses, 2.5 mg and 5 mg, down to $399 and $549 per month, respectively. This is a significant cut from the previous price of $1,059. However, these lower-cost options will only be available in vials, not auto-injector pens, and must be obtained through Lilly’s telehealth platform, LillyDirect. These vials will not be covered by insurance and will not qualify for Lilly’s discount savings program. The change is intended to address supply issues and increase accessibility but may pose challenges for patients accustomed to auto-injectors.
🔹 On August 27, Illumina announced that the FDA approved its TruSight Oncology (TSO) comprehensive test, the first distributable in vitro diagnostic kit in the U.S. with pan-cancer companion diagnostic claims. This genomic profiling test analyzes over 500 genes in a patient’s solid tumor, increasing the likelihood of identifying actionable biomarkers for targeted therapies or clinical trials. The TSO comprehensive test aims to enhance access to precision oncology.
🔹 Lykos Therapeutics is facing increasing challenges as the FDA expands its investigation into the company’s MDMA-based research for post-traumatic stress PTSD. Earlier this month, the FDA rejected Lykos’ drug application for MDMA-assisted therapy for PTSD and requested another phase 3 study to gather more data. Additionally, the journal Psychopharmacology retracted three articles related to Lykos’ clinical trials due to protocol violations and unethical conduct at one study site. The FDA is now examining whether side effects were unreported in Lykos-sponsored studies and has interviewed several individuals regarding the trials.
Read more on psychedelic therapies and Lykos’ struggles here:
🔹 On August 26, Cigna announced it will remove AbbVie’s rheumatoid arthritis drug Humira from some of its preferred drug reimbursement lists in 2025, promoting the use of less expensive biosimilar versions instead. The biosimilars to be covered include Boehringer Ingelheim’s Cyltezo, Simlandi from Teva and Alvotech, and an unbranded version of Hyrimoz from Sandoz. This decision follows a similar move by CVS Health’s Caremark unit, which led to a rapid increase in the use of Humira biosimilars. Despite the introduction of 10 biosimilar versions of Humira since January 2023, AbbVie has largely retained its market share by securing favorable positions on insurance drug coverage lists. Humira, which had peak sales of $21.2 billion in 2022, faces reduced market dominance as pharmacy benefit managers like Cigna and CVS push for cheaper alternatives.
🔹 On August 26, MBX Biosciences has filed for an IPO to raise funds to advance its drug development programs. The biotech company, based in Indiana, aims to use the IPO proceeds to move its lead candidate, MBX 2109, into phase 3 clinical trials. MBX 2109 is a peptide-based treatment for hypoparathyroidism designed for once-weekly dosing, potentially challenging Ascendis Pharma’s daily treatment Yorvipath and AstraZeneca’s phase 3 candidate, eneboparatide. Additionally, MBX plans to advance an obesity candidate, MBX 4291, a GLP-1/GIP receptor co-agonist prodrug aimed at achieving once-monthly dosing, differentiating it from competitors like Eli Lilly’s weekly tirzepatide.
🔹 On Monday, August 26, Regeneron received approval from the European Commission for its T cell engager Ordspono (odronextamab), intended to treat relapsed or refractory follicular lymphoma and diffuse large B-cell lymphoma. This approval comes five months after the FDA rejected the treatment under its accelerated approval pathway due to the immaturity of its confirmatory trials. Odronextamab is Regeneron’s first approved bispecific antibody and is targeted at patients who have undergone two or more lines of systemic therapy. Despite promising data from phase 1 and 2 trials, the FDA had sent Regeneron two complete response letters due to pending timelines for confirmatory trials, which is part of a broader issue concerning the accelerated approval pathway. Meanwhile, other companies like AbbVie, Genmab, and Roche have received FDA approval for similar treatments in the same indications over the past 15 months.
🔹 On August 26, Dexcom launched Stelo, the first over-the-counter glucose biosensor in the U.S., designed for people with Type 2 diabetes not using insulin and those with prediabetes. Stelo provides 24/7 glucose insights without the need for fingersticks, offering up to 15 days of wear and is waterproof. A two-pack (30-day supply) of sensors is available on Stelo.com for $99, or $89 per month for an ongoing subscription.
🔹 Tome Biosciences, a gene-editing biotech company based in Watertown, MA, announced on August 23rd, that it is planning to lay off 131 employees, including its CEO Rahul Kakkar and CSO John Finn. The company, which launched publicly in December 2023 after raising $213 million through series A and B funding rounds, has notified state labor officials that it will shut down by November 1, 2024. Almost all employees will be laid off, though a small number may stay on temporarily to help with the closure process. According to a spokesperson for Tome, the layoffs are partly due to a significant change in investor interest within the gene-editing field.
🔹 Two U.S. biotech companies, Zenas BioPharma and Bicara Therapeutics, filed for IPOs on the Nasdaq stock exchange on August 22 despite a challenging market environment for young biotech firms. Zenas is focused on developing obexelimab, a bifunctional monoclonal antibody for immune diseases, and is currently enrolling patients in a phase 3 trial for immunoglobulin G4-related disease. Bicara, a cancer drug developer, is in phase 1 trials with ficerafusp alfa, a bifunctional antibody for head and neck squamous cell carcinoma. Both companies have substantial venture backing, with Zenas having raised over $350 million and Bicara over $273 million.
CLINICAL TRIAL UPDATES
🔹 On August 30, Alnylam Pharmaceuticals presented additional results from the HELIOS-B study at the European Society of Cardiology’s annual meeting. The results indicate that vutrisiran reduced the risk of cardiovascular events and death compared to a placebo. While the study’s findings were promising and the company suggests vutrisiran offers additional benefits beyond those provided by Pfizer’s tafamidis, some doctors remain cautious about its potential to become the new standard of care, as there are still uncertainties.
🔹 On August 28, Novartis announced positive phase 3 results from the V-MONO study of Leqvio (inclisiran), which demonstrated significant LDL-C reduction compared to placebo and ezetimibe in patients at low or moderate risk of atherosclerotic cardiovascular disease. This study is the first to evaluate an siRNA therapy as monotherapy for LDL-C lowering.
🔹 On August 28, Palatin Technologies announced that the FDA has confirmed the acceptability of the protocols and endpoints for its remaining phase 3 trials, MELODY-2 and MELODY-3, for the drug PL9643 in treating dry eye disease. Patient enrollment is expected to begin in Q4 2024, with topline results anticipated by the end of 2025. PL9643 has shown promise in earlier trials with a rapid onset of efficacy and a strong safety profile. If successful, an NDA submission is planned for early 2026.
🔹 On August 28, Neurocrine Biosciences announced that its experimental oral drug NBI-1117568 for treating adults with schizophrenia met the primary endpoint in a mid-stage study, aiming to compete with Bristol Myers Squibb’s KarXT and AbbVie’s therapies in the growing market for schizophrenia treatments. While the lowest dose of NBI-1118568 reduced psychosis, the higher doses were not effective. NBI-1117568 was generally safe and well-tolerated at all doses studied in the phase 2 clinical trial, and the company hopes advance the drug to a phase 3 trial in early 2025. Following the announcement, Neurocrine’s stock fell 18% premarket, suggesting that investors may have expected stronger results.
🔹 On August 28, Jeune Aesthetics, a subsidiary of Krystal Biotech, announced positive interim phase 1 results for KB301, a gene therapy designed to increase collagen levels to treat aging skin. In the study, KB301 showed significant improvements in reducing wrinkles and enhancing skin quality in both the decollete and lateral canthal regions, with high subject satisfaction and no serious side effects. The company plans to advance KB301 to phase 2 trials next year for dynamic wrinkles of the decollete. Further data will be shared in upcoming conferences.
🔹 On August 28, Neurocrine Biosciences reported positive phase 2 trial results for NBI-1117568, an oral drug for schizophrenia. The 20 mg dose met the primary endpoint, showing a statistically significant 7.5-point improvement in PANSS total scores compared to placebo at six weeks. The drug also showed significant improvements in additional symptom scales and was well tolerated, with no significant weight gain or serious side effects. These results support advancing to phase 3 trials in early 2025.
🔹 On August 27, Invivyd announced positive results from an exploratory analysis of the CANOPY phase 3 clinical trial for their monoclonal antibody, pemivibart (PEMGARDA™), aimed at pre-exposure prophylaxis of COVID-19. The study found that pemivibart reduced the relative risk of symptomatic COVID-19 by 84% compared to a placebo in immunocompetent individuals, with 1.9% of pemivibart recipients contracting COVID-19 versus 11.9% in the placebo group. In immunocompromised participants, 3% contracted COVID-19, suggesting potential protection. The safety profile of pemivibart remained consistent with previous data, showing mainly mild to moderate side effects, although some severe allergic reactions were noted.
🔹 On August 22, Jazz Pharmaceuticals announced that their phase 3 trial in Japan, which evaluates the safety and efficacy of cannabidiol oral solution, Epidiolex, for treating seizures in pediatric patients with Lennox-Gastaut syndrome, Dravet syndrome, or tuberous sclerosis complex, did not meet its primary efficacy endpoint. Despite this, the company reported that some numeric improvements were seen in both primary and secondary outcomes, and no new safety concerns were noted. The trial is ongoing to gather more data, and Jazz plans to discuss a potential new drug application with Japanese regulatory authorities.
SCIENCE SPOTLIGHT
🔹 A study published in the Lancet on August 24 examined the results of the SELECT trial that examined the effects of semaglutide, a GLP-1 receptor agonist, on cardiovascular outcomes in patients with obesity and atherosclerotic cardiovascular disease. Results showed that semaglutide significantly reduced the risk of major adverse cardiovascular events and heart failure-related outcomes compared to placebo, regardless of heart failure subtype.
🔹 A study by researchers from Penn State University and Stanford published in Science suggests that cancer drugs targeting the enzyme indoleamine-2,3-dioxygenase 1 (IDO1) could be repurposed to treat neurodegenerative disorders like Alzheimer’s disease. In mouse models of Alzheimer’s disease, inhibiting IDO1 restored brain metabolism in astrocytes, improved glucose metabolism, and enhanced memory and cognitive functions. IDO1 inhibitors, already in development for cancers such as melanoma and leukemia, may help address metabolic dysfunctions in Alzheimer’s disease by blocking the kynurenine pathway.
🔹 On Saturday, August 24, it was announced that Anthony Fauci, the former head of the National Institute of Allergy and Infectious Diseases and chief medical advisor to the president, is recovering from a West Nile virus infection. For more information on West Nile virus, check out this week’s deep dive on Biotech Blueprint:
🔹 New study published in JAMA showed that long COVID symptoms differ between children and adults, and even among different age groups of children. For children aged 6-11, common symptoms include memory issues, stomach pain, and phobias. Adolescents aged 12-17 often experience symptoms like changes in smell or taste, fatigue, and headaches. These findings indicate that Long COVID can manifest uniquely in children, which requires tailored clinical attention.
ON THE HORIZON
🔹 For September 2024 PDUFAs, see last week’s newsletter:
🔹 Wells Fargo Healthcare Conference: September 4-6, 2024 (Boston, MA)
🔹 Morgan Stanley 22nd Annual Global Healthcare Conference: September 4-6, 2024 (New York, NY)
🔹 Wainwright 25th Annual Global Investment Conference: September 9-11, 2024 (New York, NY)
Happy Friday, thanks for reading and have a great long weekend!
DISCLAIMER: This content is for informational purposes only. It should not be taken as legal, tax, investment, financial, or other advice. The views expressed here are my own and do not reflect the opinions of any company or institution.
DISCLOSURE: I have no business relationships with any company mentioned in this article.







